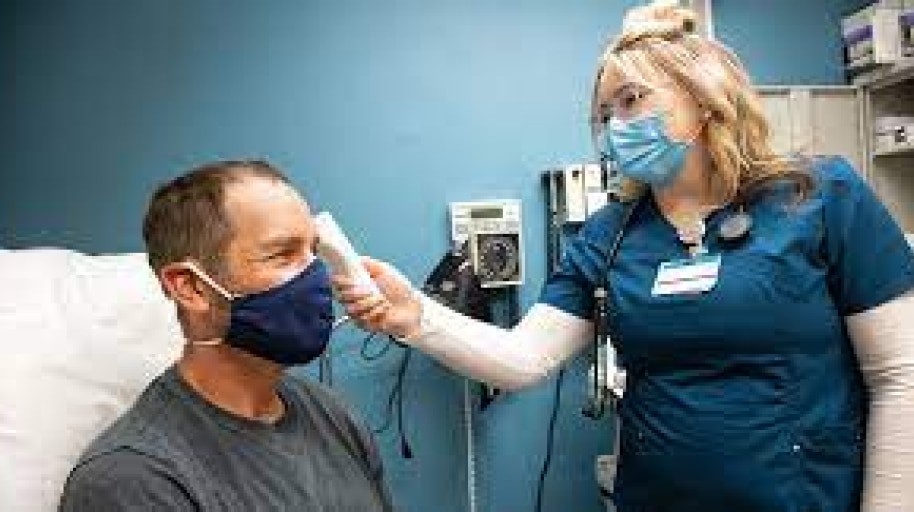
BD manufactures and sources product from multiple locations around the world. All of our global manufacturing and distribution centers are operational at this time, with the vast majority of critical-to-COVID sites operating at or near full capacity. In countries where local governments have imposed restrictions to minimize the spread of coronavirus, BD facilities continue to operate with measures in place to ensure business continuity and minimize risk of disruption to our customers.
BD continues to work closely with our key suppliers around the world that provide raw materials and components to BD manufacturing plants. We have implemented business continuity measures to mitigate the risk of potential supplier disruption, including partnering with local governments to seek “essential business” exemptions for key suppliers where necessary.
BD continues to have sufficient raw material and component inventory for the majority of products considered critical-to-COVID. Where materials or components are deemed at risk, BD activates any number of pre-approved contingency plans, including those for:
- Increased safety stock and expedited air shipments
- Seeking alternative suppliers
- Redeploying raw materials and/or finished goods from other parts of the BD network
- Placing at-risk products on customer allocation to prevent stockpiling
- Partnering with our global transportation providers and port authorities to move product through alternate routes.