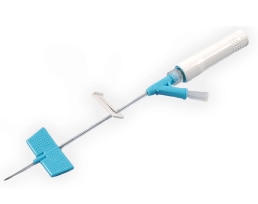
BD Saf-T-Intima™ Safety System is an over-the-needle, IV catheter. It features radiopaque BD Vialon™ Catheter Material, a needle attached to a wire stylet, wings for assisting with insertion and for catheter securement, integrated extension tubing, clamp, straight or Y-adapter, pre-attached BD® PRN Adapter, vent plug (Y-adapter only) and needle shield. The needle and catheter are protected by a needle cover. The closed system is designed to keep blood contained within the device throughout the insertion process. The needle is passively protected when it is removed.

- Overview
- Products & Accessories
- Resources
Pre-attached extension tubing offers a closed system to minimize blood exposure during cannula insertion.
BD Vialon™ Catheter Material
Softens, enabling longer dwell time and reducing the chance of phlebitis up to 69% compared to an FEP catheter.1-3
Removable adapter
A removable PRN Adapter allows the use of an ISO-compliant needle-free connector.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
1. Gaukroger PB, Roberts JG, Manners TA. Infusion thrombophlebitis: a prospective comparison of 645 Vialon and Teflon cannulae in anaesthetic and postoperative use. Anaesthesia and Intensive Care. 1988;16:265-271.
2. Kus B, Buyukyilmaz F. Effectiveness of Vialon biomaterial versus Teflon catheters for peripheral intravenous placement: A randomized clinical trial. Jpn J Nurs Sci. 2020;e12328. https://doi.org/10.1111/jjns.12328.
3. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Annals of Internal Medicine. 1991;114:845-854.
BD-112221 (01/24)
