UK


- Overview
- Best Practices
- EIFU & Resources

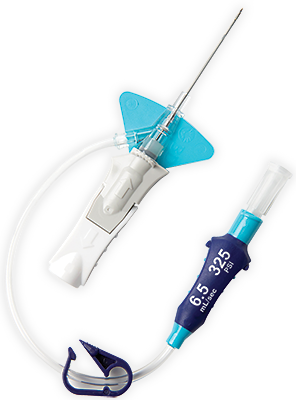
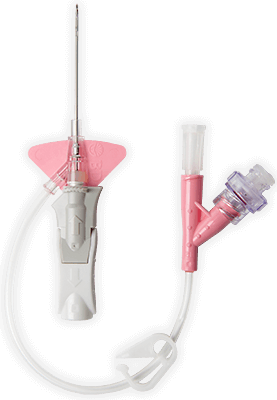
BD Vialon™ biomaterial
Proprietary BD Vialon™ biomaterial softens up to 70% in the vein, enabling longer dwell times 3† and reducing the chance of mechanical phlebitis by up to 50%.3†

BD Instaflash™ needle technology
Provides quick blood visualization that may help improve insertion success and therefore reduce insertion attempts.

Built-in stabilisation platform‡
Reduces dislodgement by 84%2§ and complies with the guidelines for catheter stabilisation set by the Infusion Nurses Society (INS)4 and Centers for Desease Control (CDC).5

Pre-attached extension tubing
Pre-attached extension tubing offers a closed system to minimise blood exposure during catheter insertion*2 meeting the INS standards.4
Reduces complications
Integrated extension tubing and stabilisation platform‡ is designed to reduce manipulation and movement at the site and has been shown to reduce dislodgement2§ and phlebitis by up to 50%.1,2
Lessens blood exposure risks
98% reduced blood exposure during insertion due to the BD Nexiva IV catheter pre-assembled systems.2*
BD Nexiva IV catheter dwells longer
In a randomised study comparing the BD Nexiva IV catheter to an open catheter system, the median dwell time for BD Nexiva IV catheters was six days versus four days for the open system.1
BD Nexiva IV catheter preserves insertion sites
By preserving sites for longer, the BD Nexiva IV catheter helps patients get the medication they need as scheduled, potentially decreasing their length of stay.1,2
May help reduce cost and delays in treatment
In a 2014 clinical study, the longer dwell time (6 days)|| of the BD Nexiva™ IV catheter led to cost reductions of up to €786,257.05 per year per 1000 beds compared with an open system.1**
BD Nexiva™ Single Port instructional video
Increased Catheter Stabilisation
Catheter stabilisation is recognised as an intervention to decrease the risk for complications and may be advantageous in preventing catheter-related bloodstream infections (CRBSIs).4,5
Integrated Configuration
The Infusion Nurses Society recommends limiting the use of add-on devices to reduce the potential for contamination, additional manipulation, and disconnection.4
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
*Compared to an open system.
**Results and savings may vary for other institutions.
†Compared with an FEP catheter.
‡When used with a BD Nexiva™ specially designed 3M™ Tegaderm™ IV site securement dressing.
§Compared with B. Braun Introcan Safety® catheter with Bard Statlock® IV Ultra stabilization device.
||Compared with 96 hours in an open system.
¶Compared with a non-blood control catheter.
References
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Ann Intern Med. 1991;114(10):845-854.
- Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. 2021, Infusion Therapy Standards of Practice, 8th Edition. Journal of Infusion Nursing. Section 6 (S108). https://doi.org/10.1097/nan.0000000000000396
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16.
BD-37378 (09/2021)