UK


- Overview
- EIFU & Resources


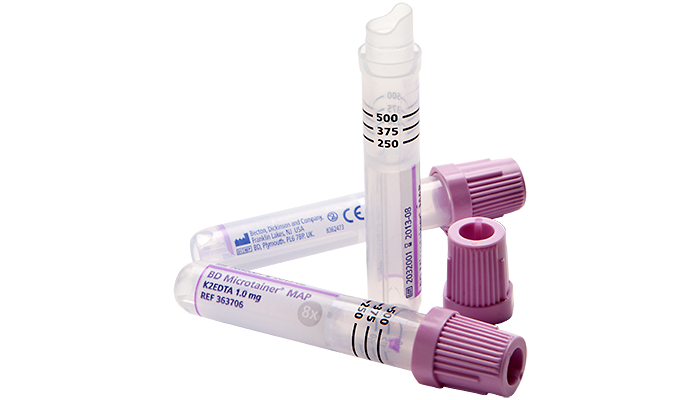
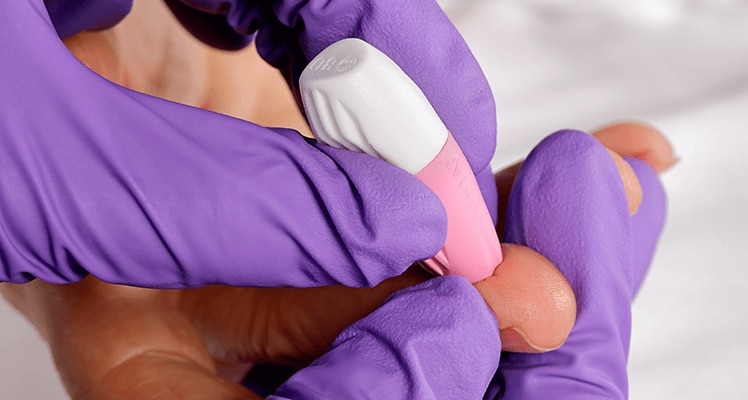
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
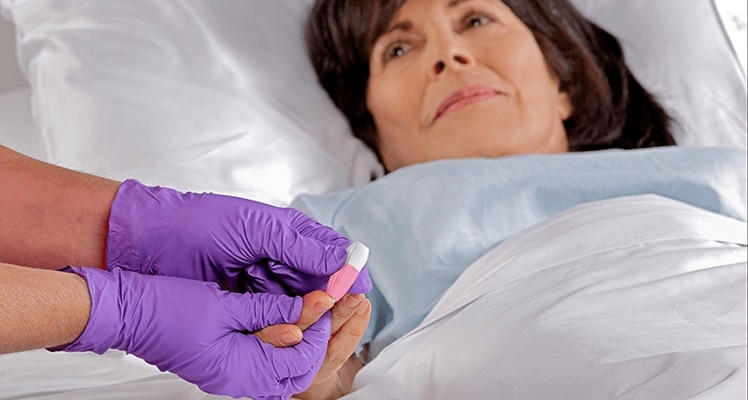
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Please note, not all products, services or features of products and services may be available in your local area. Please check with your local BD representative.
Notes
* Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS): Procedures and Devices for the Collection of Diagnostic Blood Specimens by Skin Puncture; Approved Standard (Sixth Edition). Wayne, PA, USA, 2008: Document H4-A6.
This medical device is CE marked in compliance with European Medical Device Directive 93/42/EEC and is CE certified by NSAI - National Standards Authority of Ireland (Notify Body Number = 0050 for BD Microtainer® Contact-Activated Lancet (CAL) and BD Microtainer® Quikheel™ Lancet)
BD-18634