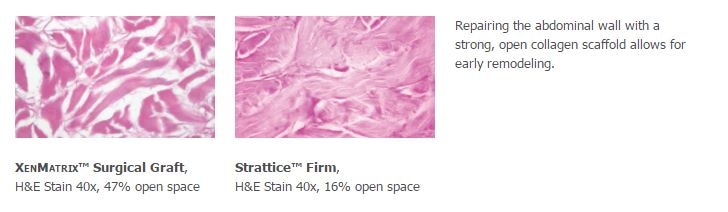
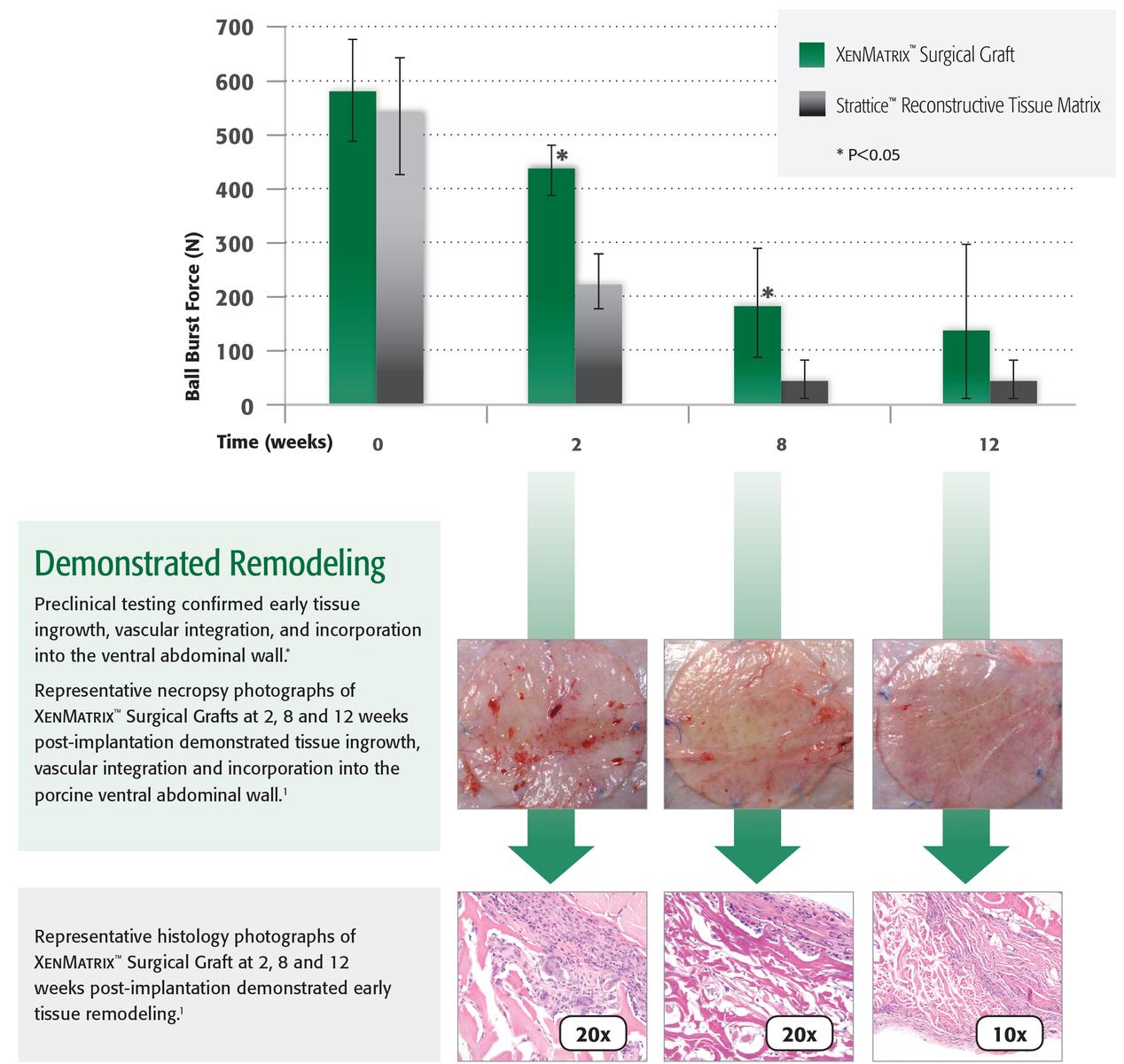
1. Preclinical data on file, results may not correlate to clinical performance
2. Deeken CR, Eliason B, Pichert M, Grant S, Frisella M, Matthews B. Differentiation of Biologic Scaffold Materials Through Physiomechanical, Thermal and Enzymatic Degradation Techniques. Ann Surg 2012. Mar; 255(3):595-604.
Indications
Intended for implantation to reinforce soft tissue where weakness exists and for surgical repair of damaged or ruptured soft tissue, including: abdominal plastic and reconstructive surgery; muscle flap reinforcement; hernia repair including abdominal, inguinal, femoral, diaphragmatic, scrotal, umbilical, and incisional hernias.
Contraindications
XenMatrix™Surgical Graft should not be used on patients with known sensitivity to porcine products. Not for reconstruction of cardiovascular defects. Not for reconstruction of central nervous system or peripheral nervous system defects. Use of this product in applications other than those indicated has the potential for serious complications.
Warnings
If an infection develops, it should be treated aggressively. An allergic reaction, which is unrelated to other therapy, is an indication to consider removal of XenMatrix™ Surgical Graft.
Precautions
Place device in maximum possible contact with healthy, well-vascularized tissue to promote cell ingrowth and tissue remodeling. When unable to close skin over the XenMatrix™ Surgical Graft, ensure that the implant remains moist. Avoid drying of the implant through “continued suction devices” as this may negatively impact the performance of the implant. Only physicians qualified in the appropriate surgical techniques should use this surgical graft. The surgeon should thoroughly understand the surgical procedure and the performance characteristics of the surgical graft.
Adverse Reactions
Potential complications with the use of any prosthesis may include, but are not limited to, allergy, seroma, infection, inflammation, adhesion, fistula formation, hematoma and recurrence of tissue defect.
Please consult package insert for more detailed safety information and instructions for use.