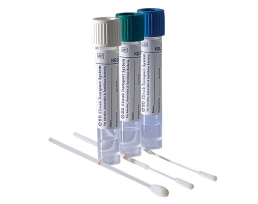
The BD ESwab™ collection and transport system helps collect clinical specimens containing aerobic, anaerobic and fastidious bacteria from the collection site, and transport them to the testing laboratory. In the lab, BD ESwab™ specimens are processed using standard clinical lab operating procedures for bacterial culture. The unique design of the flocked swab ensures optimal elution of the specimen into the transport medium.

- Overview
- Products & Accessories
- EIFU & Resources
The BD ESwab™ system is 1 mL of modified liquid Amies medium packaged with a nylon flocked swab.
The BD ESwab™ system provides 1 mL of sample suspension for Gram stain and multiple culture analyses.
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017