*No mesh is indicated for use in infected or contaminated fields.
Let’s talk about adding Phasix™ Mesh to your practice.
Sales Form
Please fill out the form and a sales representive will get in touch with you.


Supported by 5-year data,1-3 more than 35 clinical studies 4 and over 185,000 implants globally, 5 Phasix™ Mesh provides a strong, reliable repair.
PhasixTM Mesh is composed of poly-4-hydroxybutyrate (P4HB), providing critical strength during the initial healing phase with rapid tissue ingrowth and vascularization through its open-pore monofilament structure.6,7
PhasixTM Mesh gradually and predictably degrades within 12 to 18 months leaving behind a durable, functional repair with over 3x the strength of the native abdominal wall.8
After Phasix™ Mesh remodels, a strong, durable repair remains—demonstrating low recurrence at 5 years,1 lower risk of mesh-related complications9 and improved quality of life.10
P4HB gradually and predictably resorbs within 12-18 months, degrading into its 4HB monomer—a natural human metabolite found in the brain, heart, liver, kidney and muscle that is eliminated from the body via hydrolysis.1
Healthy tissue ingrowth1-3
Long-term strength and predictable durability1,4,5
Promising results in the presence of bacteria6 6-8*
*No mesh is indicated for use in infected or contaminated fields.
Preclinical and in vitro testing have shown that Phasix Mesh rapidly incorporates while the body naturally initiates an early "repair" response by preferentially up-regulating the anti-inflammatory macrophage.9,10
Preclinical data suggests that an early up-regulation in anti-inflammatory macrophages leads to a regenerative repair while other materials preferentially up-regulate the pro-inflammatory macrophage leading to fibrosis and encapsulation.11-13
As P4HB is remodeled, the body naturally responds by producing antimicrobial peptides (AMPs).9,10 Traditionally, immunology studies link AMPs to fighting bacteria.14,15
As Phasix™ Mesh remodels, it is replaced with functional tissue1-3—leaving behind a durable repair with over 3x the strength of the native abdominal wall.1

3x the strength of the native abdominal wall at 52 weeks1*
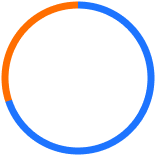
70% of PhasixTM Mesh strength is still retained at 12 weeks
*Preclinical and in vitro data on file. Results may not correlate to clinical performance in humans.
Proven clinical outcomes at 5 years1-3
40+ Clinical studies4
Over 185,000 implants globally5
More than 3,000 patients studied4
In a longitudinal 5-year follow up of 73 complex abdominal wall reconstruction patients, Phasix™ Mesh demonstrated lower recurrence, SSI and complication rates compared to Strattice™ Tissue Matrix.1
In this prospective, multi-institutional study evaluating P4HB VIHR in comorbid patients with CDC Class I wounds, five-year outcomes following VIHR with P4HB mesh were associated with infrequent complications and durable hernia repair outcomes.2
In a longitudinal 5-year follow up of 43 ventral hernia repair (VHR) patients, reported quality of life following VHR with Phasix™ Mesh improved immediately and continued to improve 5 years following repair.3
Sponsored by BD, HerniaU is a dynamic online resource for hernia repair education events, including live surgeries, case studies, lectures and comprehensive cutting edge surgical training courses.
Through live online events, the BD Hernia Surgical Education program is committed to providing updated information on advanced soft tissue repair technologies and techniques.
Connect with our Medical Information (MI) team of surgeon consultants via email (medical.services@bd.com) or phone (800.562.0027) for answers to specific technical and clinical questions on BD hernia repair products and related procedures.
Sign up to be listed in the Surgeon Locator at HerniaInfo.com.
The BD Soft Tissue Repair Reimbursement Hotline is available to answer your questions.
The hotline can be reached via phone or email.
(800)614-7965 | reimbursementsupport@bd.com
Any email or voice mail will be returned within 48 business hours. For information on physician billing, please contact your respective professional society.