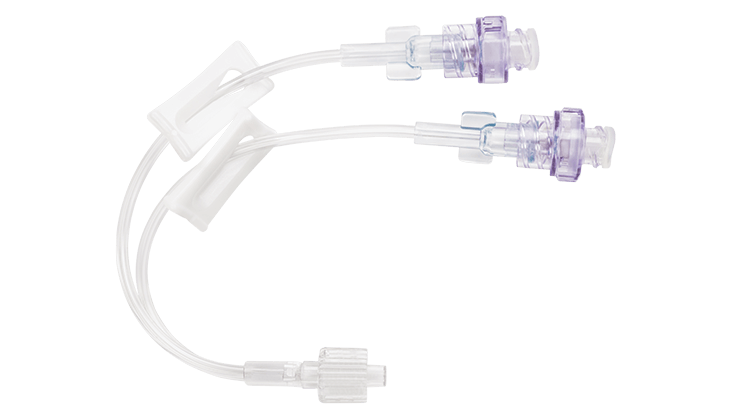
The BD Q-Syte™ needle-free connector has a split septum design that adheres to guidelines for reducing CRBSIs set by the Centers for Disease Control and Prevention (CDC) and the International Nosocomial Infection Control Consortium (INICC).
Q-Syte™ extension sets
The BD Q-Syte™ connector is a high-flow connector that aligns with CDC and INICC design preferences to reduce infections.



- Overview
- Products & Accessories
- EIFU & Resources
1. Rupp ME, Sholtz LA, Jourdan DR, et al. Outbreak of bloodstream infection temporally associated with the use of an intravascular needleless valve. Clin Infect Dis. 2007;44:1408-1414.
2. Salgado CD, Chinnes L, Paczesny TH, Cantey JR. Increased rate of catheter-related bloodstream infection associated with use of a needleless mechanical valve device at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2007;28:684-688.4.
1. Rupp ME, Sholtz LA, Jourdan DR, et al. Outbreak of bloodstream infection temporally associated with the use of an intravascular needleless valve. Clin Infect Dis. 2007;44:1408-1414.
2. Salgado CD, Chinnes L, Paczesny TH, Cantey JR. Increased rate of catheter-related bloodstream infection associated with use of a needleless mechanical valve device at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2007;28:684-688.4.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1. Rupp ME, Sholtz LA, Jourdan DR, et al. Outbreak of bloodstream infection temporally associated with the use of an intravascular needleless valve. Clin Infect Dis. 2007;44:1408-1414.
2. Salgado CD, Chinnes L, Paczesny TH, Cantey JR. Increased rate of catheter-related bloodstream infection associated with use of a needleless mechanical valve device at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2007;28:684-688.4.




