1. Surgeon survey. N=22. Data on file. Preclinical testing. Results may not correlate to clinical performance.
2. Data on file.
3. Time test compared to biologic mesh. Preclinical testing. Data on file. Results may not correlate to clinical performance.
4. Data on file. Based on preclinical testing. Results may not correlate to clinical performance in humans.
5. In preclinical testing compared to Bio-A®, Vicyrl® Mesh, and OviTex™ Resorbable Mesh. Data on file. Results may not correlate to clinical performance in humans.
Indications
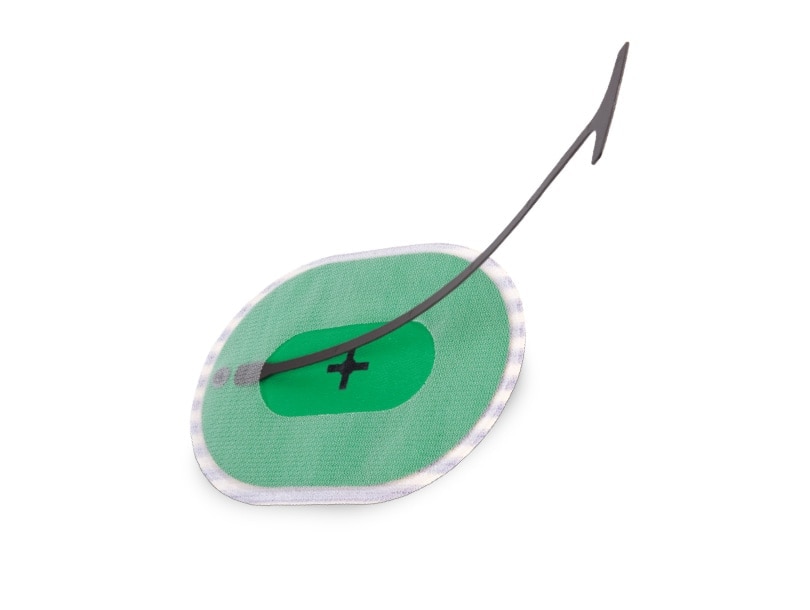
Phasix™ ST Mesh with Open Positioning System is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as for the repair of hernias. The open positioning system is intended to facilitate the placement, positioning and fixation of the mesh during open ventral hernia repair.
Contraindications
Because the mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
1. The mesh is the only implantable component of the device. The accessory (positioning guide and handle) must be removed from the patient and appropriately discarded. It is not part of the permanent implant.
2. Device manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this device in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
3. Ensure proper orientation; the coated side of the device should be oriented against the bowel or sensitive organs. Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera (reference Surface Orientation section).
4. Deviation from recommended instructions and/or procedural steps within this IFU may result in disruption or delamination of the hydrogel barrier of the mesh. Hydrogel disruption or delamination may cause an unexpected increase in adhesion formation in the area where it is disrupted.
5. The safety and effectiveness of the mesh in bridging repairs have not been evaluated or established.
The safety and effectiveness of the mesh in the following applications have not been evaluated or established:
- Pregnant women
- Pediatric use
- Neural and cardiovascular tissue
- Presence of malignancies in the abdominopelvic cavity
7. Do not apply sharp, pointed, cautery devices, or ultrasonic tools (such as scissors, needles, tackers, diathermic tools, etc.) to the accessory (positioning guide and handle).
8. Do not cut or reshape the Phasix™ ST Mesh with Open Positioning System, as this could impact its effectiveness.
9. To ensure a strong repair, the mesh should be secured with tacks or sutures through the anterior P4HB mesh or full mesh. Suturing or tacking on edge of mesh alone is not recommended.
10. To prevent recurrences when repairing hernias, the mesh must be large enough to provide sufficient overlap beyond the margins of the repair/primary closure. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
11. The use of any synthetic mesh in a contaminated or infected wound could lead to fistula formation and/or extrusion of the device and is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
Precautions
1. Only physicians qualified in the appropriate surgical techniques should use this device. Users should be familiar with strength and mesh size requirements. Improper selection, placement, positioning and fixation of the mesh can cause subsequent undesirable results.
2. Care should be taken not to damage or nick the accessory (positioning guide and handle) during fixation.
Adverse Reactions
In preclinical testing, the mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resol ved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion erosion, migration, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and instructions for use.