CapSure™ provides surgeons the confidence they desire through strong and reliable fixation.


- Overview
- Products & Accessories
- EIFU & Resources
Traditional challenges in permanent fixation for hernia repair
Permanent fixation devices facilitate a strong long-term repair but may be associated with some challenges:
- Clinical complications from exposed metal points including adhesions to fastener
- Difficulties securing large pore mesh may impact hernia repair outcomes
- Deployment challenges and device reliability issues can disrupt procedures
Bard has redefined permanent fixation
Covered
- Smooth polyetheretherketone (PEEK) cap eliminates exposed metal tip and helps minimize adhesions to the fastener*
Strong
- Fixates into Cooper’s ligament and underlying structures, similar to ProTack™
Reliable
- Comfortable handle and easy to deploy trigger
- Consistent fastener deployment and depth of tissue purchase
- Reliable, secure fixation regardless of mesh pore size
- Improved fixation with large pore meshes due to larger cap surface area of fastener versus ProTack™
*Preclinical data. Results may not correlate to the performance in humans

316L stainless steel
- 316L stainless steel is a surgical stainless steel commonly used in biomedical implants that are put under pressure including bone screws and prostheses
Smooth PEEK cap
- Cap is made from polyetheretherketone (PEEK). PEEK is an inert organic thermoplastic polymer, considered an advanced biomaterial
PEEK is used in many medical implants including dental implants, heart valves and stents, and joint prostheses
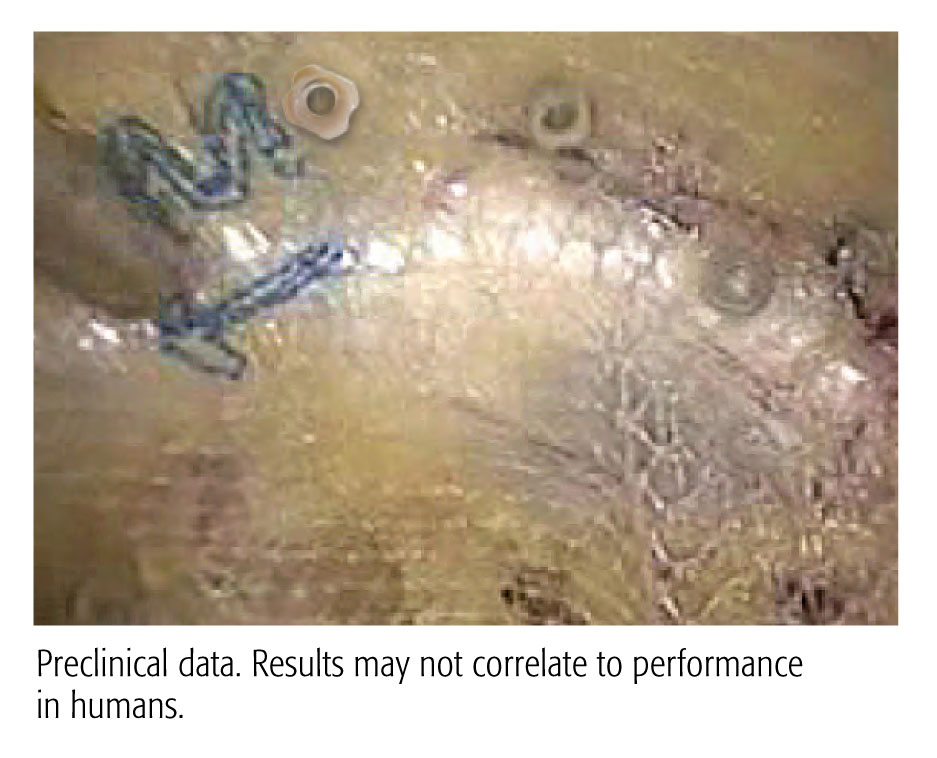
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
Precautions
Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener. Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
BD-14700
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
Precautions
Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener. Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
Precautions
Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener. Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
