Our prefillable syringes help reduce:
- Medication errors
- Syringe reuse
- Microbial contamination
- Steps to prepare injections
- Drug waste3
BD offers pharma and biotech companies a broad range of drug delivery solutions and services to help achieve goals.

Our ready-to-administer drug delivery systems, including prefillable syringes, self-injection systems, and safety and shielding devices, help ensure smooth delivery, regardless of drug complexity, viscosity and dosing volume.
Trusted Expertise
70%
Providing systems for 70% of top 100 pharmaceutical companies around the world1
500 companies
Manufacturing containers for more than 500 pharmaceutical and biotech companies worldwide2
100 molecules
Leveraging experience with hundreds of molecules spanning a broad range of therapeutic areas

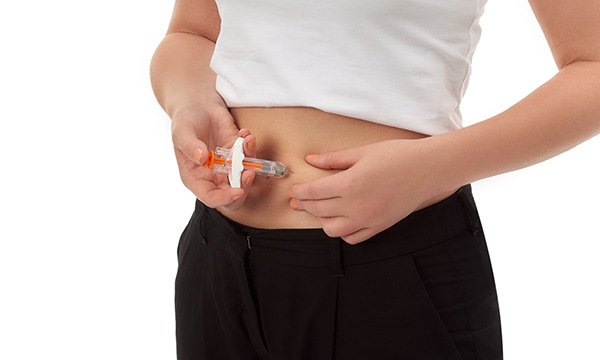
BD drug delivery systems give patients confidence, freedom and safety—appreciated benefits when it comes to receiving the medications they need. Learn how these benefits support adherence.


BD closely partners with you to fully understand the specific characteristics and requirements of your drug and your need. We then help you select and validate the optimal container and delivery system for it, leveraging our product features and designs to ensure our solutions fits your needs.
Providing experience that counts
Applying years of experience with hundreds of drug products, our dedicated Technical Services team draws from a product portfolio designed for varying drug properties and requirements to select and risk mitigate your prefillable syringes, self-injection systems, and safety and shielding solutions. We offer services to assist with formulation development, to predict and assess drug-container interactions, and to determine delivery feasibility and system performance.
In our in-house, GMP-compliant laboratories, we advise and conduct system feasibility, performance and combination product testing with your drug. We also perform analytical chemistry testing such as stability studies and extractables and leachables.
Our dedicated Regulatory Affairs team provides customized regulatory advice and documentation in alignment with worldwide filing requirements to support you in your registration process.
Our portfolio is so extensive that whatever your drug product, we offer a solution that will optimally administer it.




We are equipped to meet your production and supply needs through our reliable supply chain system, dual sourcing of major components, safety stock, ability to minimize unexpected events and high-capacity manufacturing.
BD maintains seven standardized plants worldwide, ensuring consistent quality and reporting. On a case-by-case basis, we take a customized approach to organize customer audits and site validations.
BD continuously invests in our technology and infrastructure to deliver best-in-class product performance and services. Supported by our reliable and agile manufacturing network and dedicated Centers of Excellence, we are able to develop next-generation pharmaceutical systems to meet even the most rigorous demands in this rapidly evolving industry.
At BD, we’re committed to partnering with you to help solve your medication management challenges, so you can focus on delivering quality care. Are you ready to connect?
Notes
References
About
BD provides industry-leading needle technologies, prefillable syringes, safety and shielding systems, and self-injection systems for pharmaceutical and biotech companies across the globe.

Needle technologies
Take technical innovation to needle industrialization
BD utilizes leading-edge needle technologies to combine an optimal needle point and cannula for your drug's requirements. Working with you while offering expertise, we can select the needle from our wide range of offerings that ultimately give patients the best possible experience. We can also customize the needle gauge and length upon request.
Make a sharp choice for your needle
BD needle technologies are supported by clinical and manufacturing expertise. With BD needle technologies, customers enjoy the benefits of our clinical and manufacturing expertise.

BD offers a full suite of guidance technologies for the placement of vascular access devices.

BD products for IV care and maintenance help prevent catheter related complications.

Our expanded portfolio of industry leading vascular access devices spans the vascular access continuum.
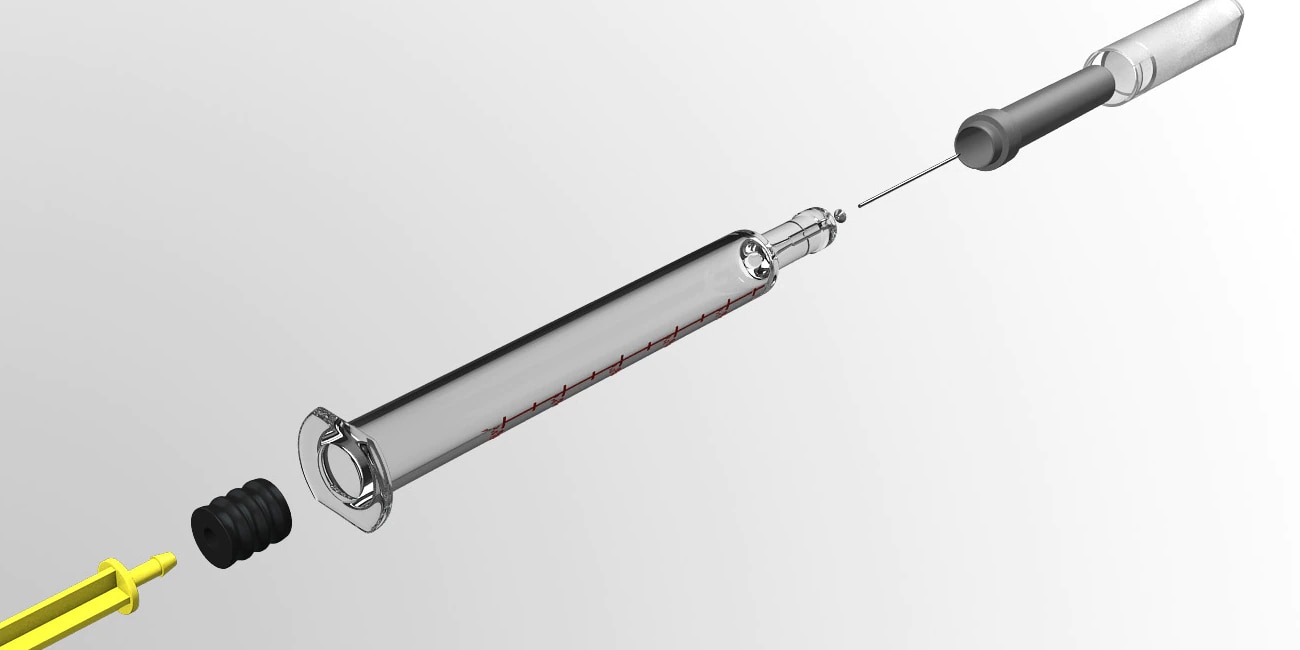
Prefillable syringe systems
Let us focus on the details, down to the molecule
Whether for biologics, vaccines, anticoagulants, or small molecules and other specialties, our prefillable glass and polymer syringe systems can help effectively, reliably and consistently administer the drug therapy you provide. Using our resourcefulness with your input, we develop your system to optimize administration and differentiate your drug—an imperative in today's competitive marketplace.
Whatever drug therapy you provide, you gain the benefit of our expertise with how drugs interact with containers, with what primary containers are right for different drugs, and if relevant, with how to effectively integrate containers or devices.

BD offers a full suite of guidance technologies for the placement of vascular access devices.

BD products for IV care and maintenance help prevent catheter related complications.

Our expanded portfolio of industry leading vascular access devices spans the vascular access continuum.
Safety and shielding systems
Protect against injury with a full shielding solution
BD offers a wide range of safety and shielding systems that feature innovative needle shielding system technology for your injectable drug. With our proprietary systems, you can help prevent the continuously increasing risks and costs of needlestick injuries that are leading government agencies to enforce more stringent legislation.1
Self-injection systems
Innovate self-injections to enhance the patient experience
Your drug impacts patients' lives and healthcare professionals' approaches to treatment. We understand that, which is why we partner with you to develop self-injection systems that address your drug delivery concerns while incorporating design factors to meet patient needs.
We provide the flexible manufacturing, customization, drug compatibility and seamless integration that combination products require today to succeed. We help you accelerate delivery time, so you can promptly take your drug to market while reducing risk—for the benefit of your company, your customers and your partners.
A global leader in self-injection systems, BD offers a differentiated solution and a full portfolio of self-injection systems designed for the patient and developed with modular platform technology that enables us to rapidly customize your product and aims to accelerate its time to market.

BD offers a full suite of guidance technologies for the placement of vascular access devices.

BD products for IV care and maintenance help prevent catheter related complications.

Our expanded portfolio of industry leading vascular access devices spans the vascular access continuum.
About
We are committed to building partnership with you and developing product solutions that meet your needs by leveraging our innovative technologies and extensive global manufacturing

Addressing your needs
At BD, we strategically develop our solutions to mitigate risks associated with combination product development and medication administration to enhance the certainty of safe and sound drug delivery.
By partnering with BD for strategic support, you can minimize risk while avoiding delays in combination product development.
Functionality you can take comfort in Partnering with BD can help you circumvent common issues that occur in medication delivery.

Our product portfolio reduces the risk of combination product performance issues by supporting complex and challenging drug formulations, larger injection volumes and higher viscosities. Our integrated primary containers and secondary systems—integrated by design—can help you reduce development risk and improve system performance.

Challenge
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus posuere, risus ac accumsan.
How bd can help
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus posuere, risus ac accumsan.

Challenge
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus posuere, risus ac accumsan.
How bd can help
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus posuere, risus ac accumsan.

Challenge
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus posuere, risus ac accumsan.
How bd can help
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus posuere, risus ac accumsan.
System Integration
As your partner, we leverage our deep industry expertise to help enhance device related aspects of your combination product's development. Through our robust data and supportive evidence, we provide critical insights to help your combination product get to market on time.
At BD, we know that total system integration – where your drug formulation, primary container and secondary device require reliable combination to deliver dependable performance under real-life conditions – is critical to getting your drug to market quickly, efficiently and safely. To give you every advantage, we will help you anticipate and address a range of needs, from human factors aspects and use-related hazards to the complex regulatory environment.6,7
SYSTEM INTEGRATION SUPPORT
BD capabilities can support you combination development means.
Patient safety is our top priority. As such our innovation process begins with patient requirements and is enhanced by our vast experience, providing integral insights to help support the success of your drug in the market.1,4 Our design considers human factors to help your combination product provide a positive injection experience. Our advanced drug delivery systems aim to support enhanced:
Insights on patient resource links:
Meeting requirements for human factors considerations (OnDrug Delivery, 2018) >
In drug development, project delays can equal meaningful increases in costs that can impact your profitability and the viability of bringing your drug to market.5 Our goal is to help you anticipate obstacles, mitigate risks and solve for operational challenges, so your combination product meets timelines and performs under real-life conditions. BD can help augment your combination development capabilities and support efficiency through:
Launching a combination product can come with many challenges.5 As a global partner with deep expertise in drug delivery solutions and drug device combination products, BD can help you bring your drug to market smoothly and efficiently by1:
The impact of our extensive industry experience:
About
At BD, we have built our services and capabilities around a deep understanding of the drug development process and the key objectives at each stage. Knowledgeable and responsible, our team offers the many capabilities you need beyond taking your product to market.

BD PartnerPathTM program
BD PartnerPath™ Program aims to support pharmaceutical organizations that value speed to market in combination product development.1
No matter where you are in the process of combination product development, BD can help you reach your goals.
REALIZED BENEFITS
Each of our services is delivered intentionally and carefully to de-risk your development journey and accelerate the launch of your drug combination product on the market
Flexible offer
Our goal : offer services tailored to your needs
Access to scientific testing experts
Our goal : provide rapid and accurate answers to your questions
World-class container selection and validation
Our goal : develop optimal solutions for your primary container and secondary device
Combination product knowledge and testing capabilities
Our goal : ensure component and system performance consistency
Customizable protocols
Our goal : deliver relevant results addressing your specific needs
GMP-compliant labs with state-of-the-art equipment
Our goal : provide you with high-quality test results that can be leveraged in regulatory filings
Products
BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.
Supporting pharmaceutical organizations in the development process with:
*Available for select products and product systems
**Minimum purchase quantity at the lowest sterilized package configuration
‡Data and services are available for specific BD products and product configurations
Product engineering and device testing
To support customer system integration, we offer an evaluation of your delivery system functional performance testing, including:
Evaluate primary container and drug interaction
We provide predictive tools, subvisible particle analysis, Tungsten studies, and extractable and leachable studies to help:
Evaluate combination product performance
To assist in evaluating device and drug combination product functional performance for needle-based injection systems, safety systems, and autoinjector systems, we offer:
*Available for select products and product systems
When your data is scattered, your operations can be too. Connected Medication Management leverages your EMR to create a comprehensive view of inventory on hand. That means managing fewer databases and gaining visibility so you can make valuable impacts on workflows across your organization and minimize medication diversion and adverse drug events. By having connected systems and bidirectional data exchange, predictive analytics can inform critical decision-making about care needs, help control carrying costs and avoid wasted inventory.
Access to GMP production filling assembling, and labeling for not-for human-use small volumes, with services including:*±
Filling
Assembling
Labeling
*Available for select products and product systems
±Customer samples required
Supporting pharmaceutical organizations in the development process with:
*Available for select products and product systems
**Minimum purchase quantity at the lowest sterilized package configuration
‡Data and services are available for specific BD products and product configurations
Products

BD Pharmaceutical Services and Solutions are designed to help you achieve your combination product goals, from development to launch.

BD PartnerPath™ Program aims to support pharmaceutical organizations that value speed to market in combination product development.1
See below on our featured resources
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
リンク先が海外サイトの場合、そのサイトには日本では承認されていない製品または適用に関する情報が掲載されている場合があります。
第三者によって管理されているサイトの内容およびリンク先サイトの利用(プライバシー・ステートメント、コンテンツ等)については一切の責任を負いかねます。
外部サイトの利用については、そのサイトの利用条件をご確認ください。
外部サイトを利用したくない場合は、このウィンドウを閉じてください。