Medium weight monofilament polypropylene mesh on the anterior side with an absorbable hydrogel barrier based on Sepra® Technology on the posterior side for laparoscopic ventral hernia repair.
Maille Ventralight™ ST
Maille en polypropylène de poids moyen, monofilament sur la face antérieure, avec une barrière en hydrogel résorbable basée sur la technologie Sepra® sur la face postérieure, conçue pour la réparation des hernies ventrales dans le cadre d’une laparoscopie.


- Présentation
- Produits et accessoires
- Mode d’emploi en version électronique et ressources
Efficace
- La conception compacte facilite le déploiement du trocart et la fixation mécanique.
- Diverses formes (ronde, ovale, elliptique, rectangulaire) et tailles allant d’un rond de 11,4 cm (4,5 po) à un rectangle de 30,5 cm x 35,6 cm (12 po x 14 po).
- Facile à découper pour personnaliser la forme et la taille en fonction des besoins. La barrière unique en hydrogel recouvre les rebords de la maille, même après sa découpe.
Efficace
Contraction minimale démontrée dans les essais précliniques par rapport à une maille macroporeuse à barrière résorbable bien connue sur le marché
En quatre semaines, la maille Ventralight™ ST a démontré une contraction de la surface de la maille inférieure de 42 % à celle d’une maille macroporeuse à barrière résorbable concurrente.
Les résultats étaient statistiquement significatifs.*
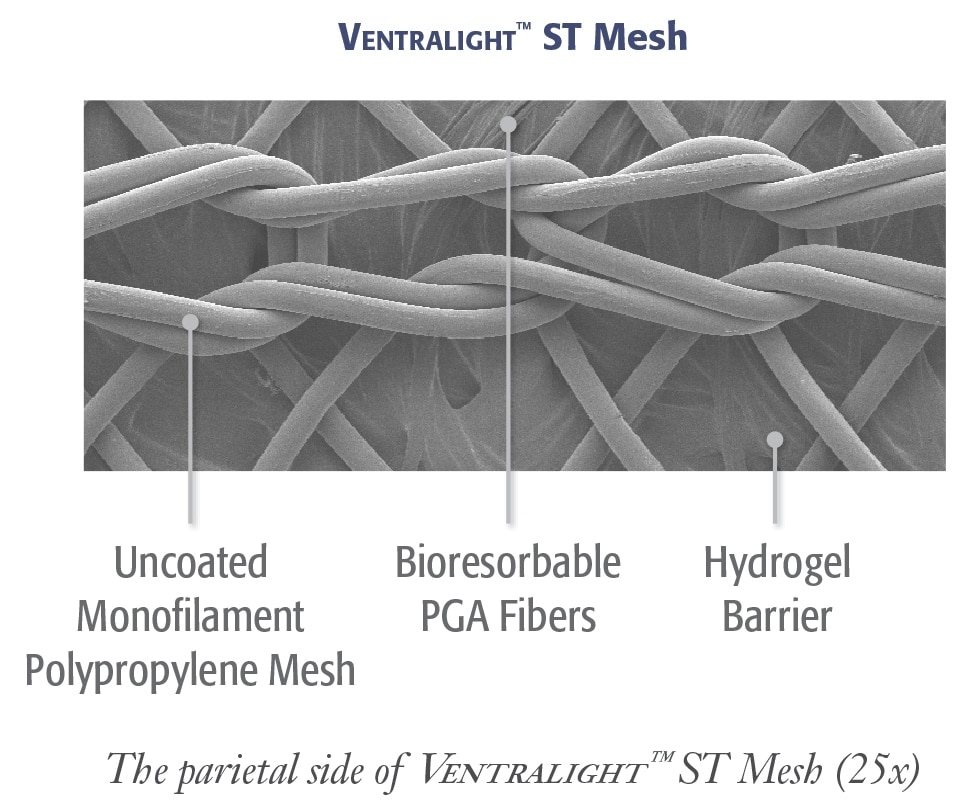
Éprouvée
Barrière résorbable Ventralight™ basée sur la technologie Sepra®
- Une barrière en hydrogel unique gonfle pour minimiser l’adhérence du tissu au côté viscéral de la maille*
- La barrière en hydrogel se résorbe en 30 jours, en assurant la protection des viscères pendant la période cruciale de cicatrisation*
Forte incorporation tissulaire éprouvée
La conception à pores ouverts du polypropylène monofilament non enduit de la maille Ventralight™ ST favorise :
- Une croissance tissulaire rapide
- Une incorporation tissulaire importante dans la paroi abdominale
- Une robuste réparation à long terme Le polypropylène non enduit favorise la majeure partie de la croissance tissulaire et du renforcement pendant les deux premières semaines suivant la pose d’une prothèse pour hernie composite.**
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
-
>strong>ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14799
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
-
>strong>ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD met à votre disposition des ressources de formation pour vous aider à améliorer vos pratiques cliniques, dans le cadre de notre objectif « faire progresser le monde de la santé ».
BD soutient le secteur des soins de santé en proposant des produits et des services de pointe pour améliorer les soins tout en diminuant les coûts. Nous organisons des événements et participons à des rencontres qui excellent à « faire progresser le monde de la santé™ ».
* Preclinical data on file at BD. Results may not correlate to performance in humans.
** Based on a preclinical study of a composite polypropylene/ePTFE hernia repair mesh.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRAINDICATIONS
- Do not use the Ventralight™ ST in infants or children whereby future growth will be compromised by use of such material.
- Do not use Ventralight™ ST Mesh for the reconstruction of cardiovascular defects.
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
- Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs. Do not place the polypropylene side against the bowel. There may be a possibility for adhesion formation when the polypropylene side is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the prosthesis. An unresolved infection may require removal of the prosthesis. Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
-
>strong>ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
