Venclose Maven™ Perforator Catheter
Streamlining Venous Reflux Disease Treatment


- Overview
- Products & Accessories
- EIFU & Resources
Venclose Maven™ Perforator Catheter
For patients with Chronic Venous Disease (CVD), the Venclose Maven™ Perforator Catheter is used for treatment of incompetent perforator veins (IPVs) through endovenous radiofrequency (RF) ablation which has been established as a CVD treatment option for more than 20 years.
While some vein catheters can be reprocessed more than once and used on different patients, the Venclose Maven™ Perforator Catheter is a single-use, sterile, minimally invasive, thermal treatment option and not a permanent implant. This device is intended for endovascular coagulation of blood vessels in patients with perforator and tributary vein reflux.
The only 360º RF solution for the treatment of incompetent perforator veins with a 0.5 cm circumferential resistive heating coil*
*As of Dec 2022

0.5 cm Resistive Heating Coil
- Treat IPVs with a modernized, circumferential heating coil vs. repeated quadrant ablations for bipolar electrodes
6F Low-Profile Design
- Small profile helps to minimize invasiveness, guidewire compatible with 0.025" or smaller GW sizes
40 cm Flexible Catheter Shaft
- Helps facilitate efficient treatment for varying vein lengths and anatomies
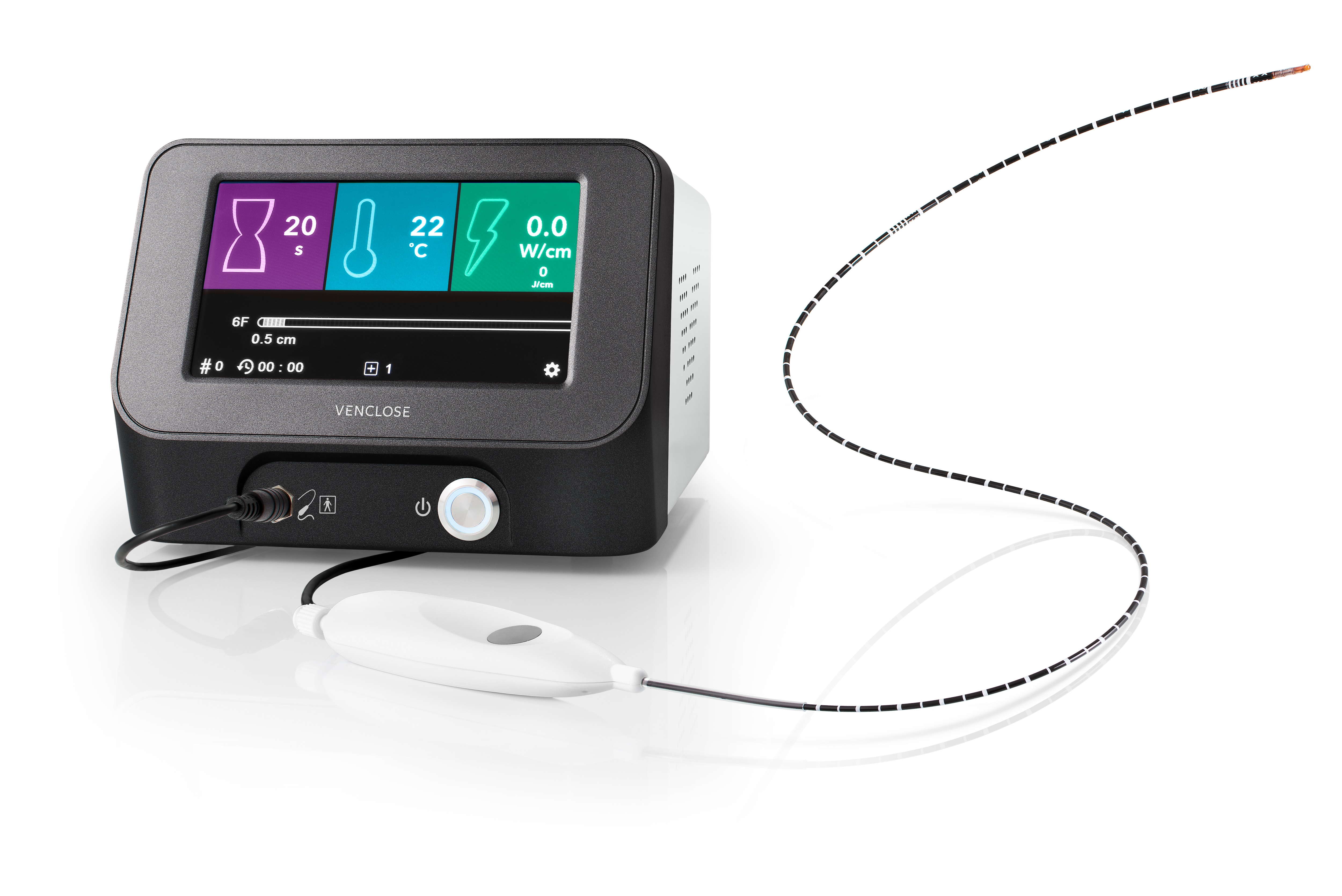
The system is equipped with a touchscreen display providing real-time procedure data to help inform physician treatment decisions.
- Compact design
- Nearly instant power up, activated by the handle or the foot pedal without having to leave the sterile field
- Simple, catheter connection port allows quick and easy catheter plug-in
- Audible tones for thermal delivery allows you to focus more on the patient, and less on the display
The Venclose Maven™ Perforator Catheter is powered by the Venclose™ RF Ablation Generator, both are intended to be used together as a system.
1 Yost ML. Chronic venous disease (CVD): Epidemiology, costs, and consequences. Beaufort, SC: The Sage Group; 2016.
2 De Maeseneer MG, Kakkos SK, Aherne T, et al. European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63:184-267.
3 Criqui MH, Denenberg JO, Langer RD, Kaplan RM, Fronek A. Epidemiology of chronic peripheral venous disease. In Bergan J, ed. The Vein Book, 1st ed. Academic Press; 2006.
4 Rice J, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347-356.
5 O'Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery(R) and the American Venous Forum. J Vasc Surg.2014;60:3S-59S.
Please consult product labels and instructions for the use of indications, contraindications, hazards, warnings, and precautions. BD, the BD Logo, and Venclose Maven are trademarks of Becton, Dickinson and Company or its affiliates. © 2022 BD. All Rights Reserved. © Illustration by Mike Austin.
BD-69485 | MK-0113.A
1 Rice J (2014). Burden of venous leg ulcers in the United States. Journal of Medical Economics. 17(5), 347-356
2 O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: Clinical Practice Guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc. Surg. 2014: 60; 35-595.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-69485 | MK-0113.A
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1 Rice J (2014). Burden of venous leg ulcers in the United States. Journal of Medical Economics. 17(5), 347-356
2 O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: Clinical Practice Guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc. Surg. 2014: 60; 35-595.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-69485 | MK-0113.A


