Maille Phasix™ ST
Échafaudage entièrement biorésorbable muni de la technologie Sepra® éprouvée


- Présentation
- Produits et accessoires
- Mode d’emploi en version électronique et ressources
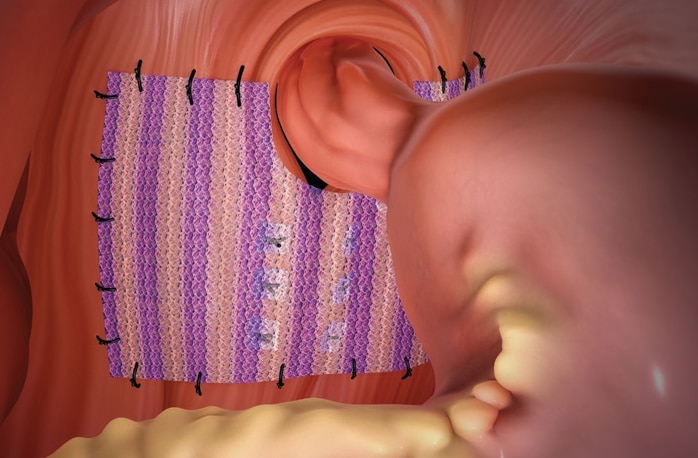
Phasix™ ST Mesh combines two market-leading technologies into one product: monofilament resorbable Phasix™ Mesh and a proven hydrogel barrier based on Sepra® technology. While the monofilament mesh supports functional healing and a strong repair, the hydrogel barrier minimizes tissue attachment to the visceral side of the mesh for intraabdominal placement.1
These meshes are available in different shapes, such as round, rectangle, and square.
For high-risk/comorbid patients, surgeons have had to choose between permanent synthetic meshes and biologic grafts—and their inherent pros and cons. Synthetic meshes can have complications that lead to mesh removal or reoperation, while biologic grafts can have accelerated degradation in the presence of bacteria which may lead to mesh failure.
Phasix™ ST Mesh handles, sutures and fixates like a synthetic mesh, while exhibiting the remodeling characteristics of a biologic mesh. It facilitates trocar deployment during laparoscopic placement.
The ST hydrogel barrier
- Minimizes risk of tissue attachment
- Has over 10 years of clinical application
- Resorbs in 30 days
- 15 clinical studies
The Phasix™ ST Mesh is a biologically derived scaffold with a hydrogel barrier for intraabdominal placement. It has been designed to provide the repair strength of a synthetic mesh and the remodeling characteristics of a biologic.
The Phasix™ ST Mesh does the following:
- Handles, sutures and fixates like a synthetic mesh
- Facilitates trocar deployment during laparoscopic placement
- Its longitudinal stripes aid with orientation and visibility during placement
STRUCTURE DU MATÉRIAU1
Il a été déterminé que les modèles à maille monofilament offrent une biocompatibilité supérieure et présentent moins de risques d’adhérence et de colonisation bactériennes.
RÉPARATION DES HERNIES1
La structure ouverte de la maille monofilament permet une intégration précoce et une réparation plus robuste.1
PROBLÈMES DE REMODELAGE AU FIL DU TEMPS1
L’intégration et l’incorporation vasculaires se poursuivent, avec une abondance de collagène mature à 52 semaines. Transfère progressivement la charge au tissu natif au fil du temps.1
TECHNIQUES POLYVALENTES1
La maille Phasix™ ST peut être placée en position intra-abdominale ou pré-péritonéale après la fermeture de l’anomalie herniaire primaire. La fermeture de l’anomalie herniaire primaire doit être réalisée avant la pose de la maille.
FERMETURE DE L’ANOMALIE HERNIAIRE
La fermeture de l’anomalie herniaire peut être réalisée par une approche ouverte ou peu invasive (c’est-à-dire laparoscopique, robotique). Des études récentes suggèrent que la fermeture de l’anomalie présente les avantages potentiels suivants (liste non exhaustive) :
• Diminution de l’espace « mort », ce qui peut réduire le risque de sérome postopératoire
• Possible contribution à la restauration d’une paroi abdominale fonctionnelle
• Possible réduction du gonflement postopératoire au niveau du site de l’anomalie herniaire
RÉSULTATS PROMETTEURS EN PRÉSENCE DE BACTÉRIES
Il n’a pas été démontré que la maille Phasix™ ST se décompose en présence de bactéries, en maintenant 100 % de sa résistance à 56 jours, contrairement aux greffons biologiques qui démontrent une dégradation accélérée en présence de bactéries.
Comme illustré ci-dessous, aucune présence de colonisation bactérienne n’a été observée dans la maille Phasix™ ou Phasix™ ST sept jours après l’inoculation dans des tests précliniques. L’autre côté du graphique présente la présence d’un abcès (matière blanche) observée avec SurgiMend®, Strattice™, Bio-A® et OviTex™. D’autres indications observées de colonisation bactérienne comprenaient un gonflement, la présence de fluides et un épaississement du tissu de la capsule.
1. Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
2. DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Indications
Phasix™ ST Mesh is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as for the repair of hernias, including hiatal hernias.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Device manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this device in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs.
Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established. The use of any synthetic mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
For hiatal hernia repair, the use of Phasix™ ST Mesh circumferentially around the esophagus is not recommended.
For hiatal hernia repair, the use of Phasix™ ST Mesh to bridge the hiatus is not recommended.
The safety and effectiveness of Phasix™ ST Mesh in the following applications has not been evaluated or established: Pregnant women, Pediatric use, Neural and Cardiovascular tissue.
Precautions
The safety and effectiveness of Phasix™ ST Mesh has not been evaluated in the presence of malignancies in the abdominopelvic cavity.
Adverse Reactions
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include esophageal erosion and dysphagia related to crural fibrosis.
Please consult package insert for more detailed safety information and instructions for use.
BD-76012
1. Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
2. DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Indications
Phasix™ ST Mesh is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as for the repair of hernias, including hiatal hernias.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Device manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this device in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs.
Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established. The use of any synthetic mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
For hiatal hernia repair, the use of Phasix™ ST Mesh circumferentially around the esophagus is not recommended.
For hiatal hernia repair, the use of Phasix™ ST Mesh to bridge the hiatus is not recommended.
The safety and effectiveness of Phasix™ ST Mesh in the following applications has not been evaluated or established: Pregnant women, Pediatric use, Neural and Cardiovascular tissue.
Precautions
The safety and effectiveness of Phasix™ ST Mesh has not been evaluated in the presence of malignancies in the abdominopelvic cavity.
Adverse Reactions
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include esophageal erosion and dysphagia related to crural fibrosis.
Please consult package insert for more detailed safety information and instructions for use.
BD met à votre disposition des ressources de formation pour vous aider à améliorer vos pratiques cliniques, dans le cadre de notre objectif « faire progresser le monde de la santé ».
BD soutient le secteur des soins de santé en proposant des produits et des services de pointe pour améliorer les soins tout en diminuant les coûts. Nous organisons des événements et participons à des rencontres qui excellent à « faire progresser le monde de la santé™ ».
1. Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
2. DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Indications
Phasix™ ST Mesh is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as for the repair of hernias, including hiatal hernias.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Device manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this device in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs.
Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established. The use of any synthetic mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
For hiatal hernia repair, the use of Phasix™ ST Mesh circumferentially around the esophagus is not recommended.
For hiatal hernia repair, the use of Phasix™ ST Mesh to bridge the hiatus is not recommended.
The safety and effectiveness of Phasix™ ST Mesh in the following applications has not been evaluated or established: Pregnant women, Pediatric use, Neural and Cardiovascular tissue.
Precautions
The safety and effectiveness of Phasix™ ST Mesh has not been evaluated in the presence of malignancies in the abdominopelvic cavity.
Adverse Reactions
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include esophageal erosion and dysphagia related to crural fibrosis.
Please consult package insert for more detailed safety information and instructions for use.