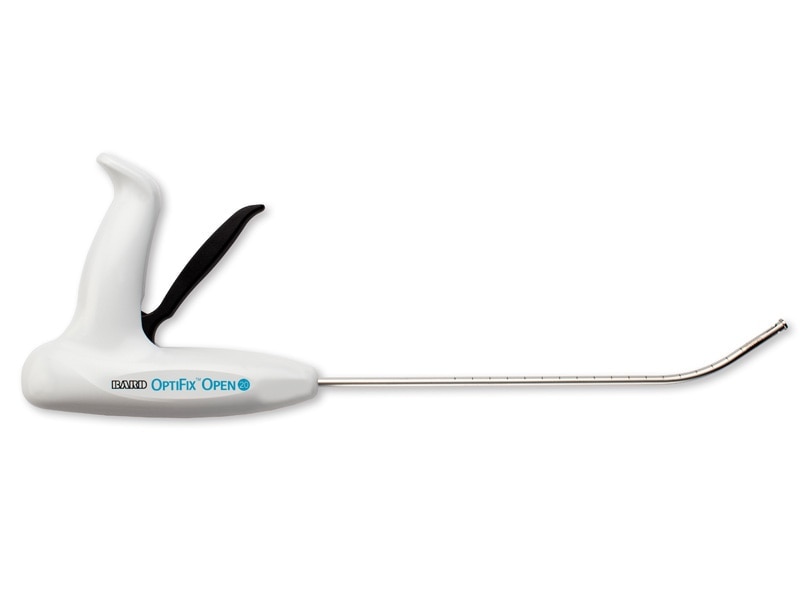
The OptiFix™ Open Absorbable Fixation System was designed for improved ease of use when fixating surgical meshes to the abdominal wall in open ventral hernia procedures by reducing limitations that exist with current laparoscopic straight devices. Combined with Ventrio™ ST, it provides a full system for open ventral hernia repair.
OptiFix™ Open Absorbable Fixation System
Advancing the Open Ventral Hernia Repair Experience

- Overview
- Products & Accessories
- EIFU & Resources
- FAQ
For Open Ventral Hernia Repair

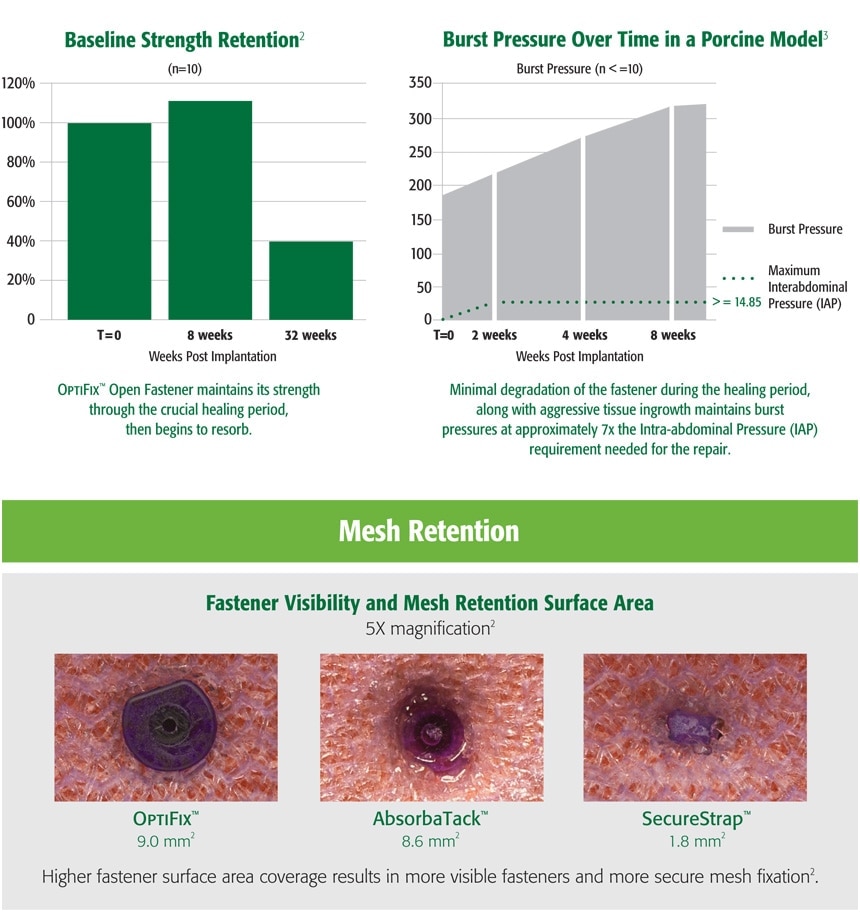
1. Preclinical data on file. Results may not correlate to clinical outcomes.
2. C. R. Bard Inc., bench data on file.
3 Preclinical data on file. Results may not correlate to clinical outcomes.
4 Survey of surgeons attending pre-clinical lab.
Results may not correlate to clinical outcomes. Data on file.
OptiFix™ Open Absorbable Fixation System
Indications
The OptiFix™ Open Absorbable Fixation System is indicated for the fixation of surgical mesh to tissues during open surgical procedures, such as hernia repair.
Contraindications
Contraindications associated with open surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage,
- Situations with insufficient ingrowth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is absorbed.
- Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the OptiFix™ Open Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 6.1 mm, the fastener head is another 0.6 mm (total 6.7 mm).
Warnings
The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use. After use, the OptiFix™ Open Absorbable Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the OptiFix™ Open Absorbable Fixation System may include, but are not limited to the following: hemorrhage; pain, edema and erythema at wound site; allergic reaction to Poly(D, L)-lactide; infection/septicemia; hernia recurrence/wound dehiscence.
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and instructions for use.
| BD-15157 |
1. Preclinical data on file. Results may not correlate to clinical outcomes.
2. C. R. Bard Inc., bench data on file.
3 Preclinical data on file. Results may not correlate to clinical outcomes.
4 Survey of surgeons attending pre-clinical lab.
Results may not correlate to clinical outcomes. Data on file.
OptiFix™ Open Absorbable Fixation System
Indications
The OptiFix™ Open Absorbable Fixation System is indicated for the fixation of surgical mesh to tissues during open surgical procedures, such as hernia repair.
Contraindications
Contraindications associated with open surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage,
- Situations with insufficient ingrowth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is absorbed.
- Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the OptiFix™ Open Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 6.1 mm, the fastener head is another 0.6 mm (total 6.7 mm).
Warnings
The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use. After use, the OptiFix™ Open Absorbable Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the OptiFix™ Open Absorbable Fixation System may include, but are not limited to the following: hemorrhage; pain, edema and erythema at wound site; allergic reaction to Poly(D, L)-lactide; infection/septicemia; hernia recurrence/wound dehiscence.
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and instructions for use.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1. Preclinical data on file. Results may not correlate to clinical outcomes.
2. C. R. Bard Inc., bench data on file.
3 Preclinical data on file. Results may not correlate to clinical outcomes.
4 Survey of surgeons attending pre-clinical lab.
Results may not correlate to clinical outcomes. Data on file.
OptiFix™ Open Absorbable Fixation System
Indications
The OptiFix™ Open Absorbable Fixation System is indicated for the fixation of surgical mesh to tissues during open surgical procedures, such as hernia repair.
Contraindications
Contraindications associated with open surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage,
- Situations with insufficient ingrowth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is absorbed.
- Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the OptiFix™ Open Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 6.1 mm, the fastener head is another 0.6 mm (total 6.7 mm).
Warnings
The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use. After use, the OptiFix™ Open Absorbable Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Adverse Reactions
Adverse reactions and potential complications associated with fixation devices such as the OptiFix™ Open Absorbable Fixation System may include, but are not limited to the following: hemorrhage; pain, edema and erythema at wound site; allergic reaction to Poly(D, L)-lactide; infection/septicemia; hernia recurrence/wound dehiscence.
Please consult product labels and inserts for any indications, contraindications, hazards, warnings, precautions and instructions for use.
