true
Echo PS™ Positioning System with Ventralight™ ST Mesh
Ventralight™ ST Mesh with a pre-attached low-profile balloon to help facilitate deployment, placement and positioning in laparoscopic ventral hernia repair.

- Overview
- Products & Accessories
- EIFU & Resources
Ventralight™ ST Mesh with a pre-attached low-profile balloon to help facilitate deployment, placement and positioning in laparoscopic ventral hernia repair.

Mesh Preparation Comes pre-attached to Ventralight™ ST Mesh
Designed to ensure that barrier protection is oriented towards viscera.
Reduces mesh preparation time and user frustration.1

Deployment
Does not require dedicated trocar.
Potentially reduces patient trauma.
Following mesh introduction, trocar is free to be used with other laparoscopic instruments.

Positioning
Technique designed to center mesh over defect.
Alleviates guesswork and facilitates centering
Compared to positioning with trans-facial sutures, Echo PS™ Positioning System:
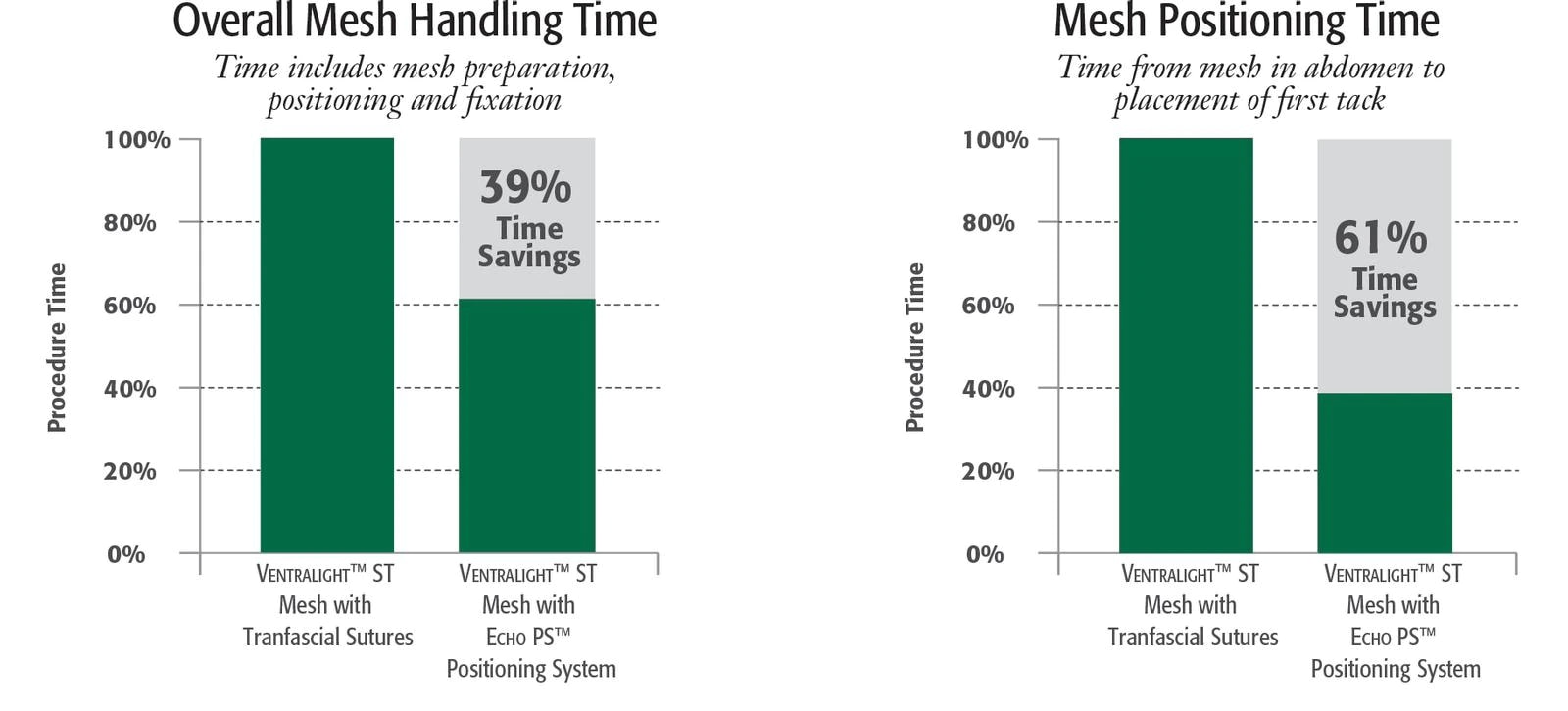
Reduces mesh positioning by over 60%1
Reduces procedure time variability by 84%1

Fixation
Requires only one set of hands.
Once positioned with the Echo PS™ Positioning System, mesh stays in place allowing the surgeon to use both hands for fixation.
No assistance or handoff needed to hold mesh in place during fixation potentially leading to increased accuracy.
Unique flexible balloon design.
Allows mesh to conform to the abdominal wall.
Occupies less intraabdominal space and is designed not get in the way of fixation.
The Echo PS™ Positioning System puts the control into the surgeon’s hands resulting in reproducible results in less time.1
- The Echo PS™ Positioning System technique eliminates the guesswork by facilitating accurate mesh centering and assisted fixation without the need for an extra set of hands.
- More than 3 years of clinical experience and a growing number of surgeons adopting the technology into their standard practice.
- Award-winning innovative design streamlines laparoscopic procedures, saving time and reducing procedure time variability by 84%.1
Mesh Handling Times and Positioning Times Charts
Click on image above to expand chart view.
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Training
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Case Studies
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
1 When compared to positioning with transfascial sutures in a preclinical study.
Date on file at C. R. Bard, Inc. Results may not correlate to performance in humans.
INDICATIONS
Ventralight™ ST Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias. Composix™ L/P Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias and chest wall defects. The Echo PS™ Positioning System is intended to be used to facilitate the delivery of soft tissue prostheses during laparoscopic hernia repair.
CONTRAINDICATIONS
Literature reports there is a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
WARNINGS
Ventralight™ ST Mesh/Composix™ L/P Mesh is the only permanent implant component of the device. The inflation adapter and syringe are to be kept external to the patient and discarded after use. The Echo PS™ Positioning System (including the balloon, all connectors, and inflation tube) is to be removed from the patient and appropriately discarded as it is not part of the permanent implant.
The Echo PS™ Positioning System should not be used with any other hernia prosthesis aside from those with which it comes pre-attached/packaged.
PRECAUTIONS
Do not trim the mesh. This will affect the interface between the mesh and positioning system.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14779
true

